Important Properties of Compounds of Copper
Important Properties of Compounds of Copper: Overview
This topic covers concepts, such as, Copper(II) Oxide, Compounds of Copper, Preparation of Copper Sulphate & Properties of Copper Sulphate etc.
Important Questions on Important Properties of Compounds of Copper
Write the Physical Properties of Copper Oxide or Cupric Oxide.
Write a balanced chemical equation for the following word equation:
A substance (A) is water-insoluble, and on bubbling gas through its suspension in water, it produces a coloured aqueous solution, forming a single product. Substance (A) may be:
Which of the following methods are not the best scientific method to test the presence of water in a liquid?
. The product and respectively are
Which of te following statements is incorrect?
Consider in the reaction, ion is ?
When solution in water is treated with concentrated it turns
While passing hydrogen over heated black color is oxidized to
Copper sulphate is prepared by blowing a current of air through Cu scrap and dilute H2SO4. Dilute HNO3 is also added
For
which of the following is correct ?
CuSO4 . 5H2O is a:
Identify A to D in following reactions
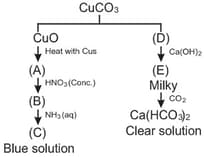
The compounds A, B, C & D may be
Which of the following is the best scientific method to test the presence of water in a liquid?
Which of the following is formed when excess of KCN is added to aqueous solution of copper sulphate ?
Which of the following is formed, when excess of is added to an aqueous solution of copper sulphate?
A black sulphide is formed by the action of H2S on
A black sulphide is formed by the action of on:
